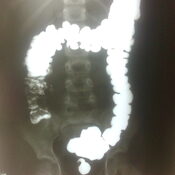
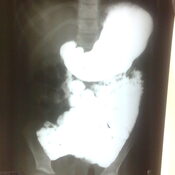
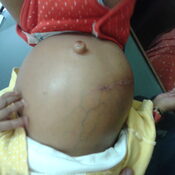
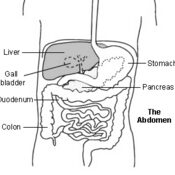
Management of Neonatal Cholestasis: Consensus Statement of the Pediatric Gastroenterology Chapter of Indian Academy of Pediatrics ,Indian Pediatrics Volume 51, 2014:203-210
VIDYUT BHATIA, *ASHISH BAVDEKAR, $JOHN MATTHAI, #YOGESH WAIKAR AND ANUPAM SIBAL
From Apollo Center for Advanced Pediatrics, Indraprastha Apollo Hospital, New Delhi; *Department of Pediatrics, King Edwards
Memorial Hospital, Pune; $Department of Pediatrics, PSG Institute of Medical Sciences, Coimbatore and #Pediatric
Gastroenterology Clinic, Care hospital, Nagpur, India.
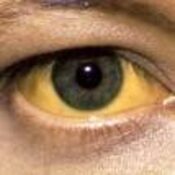
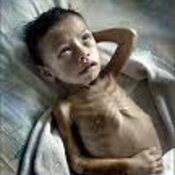
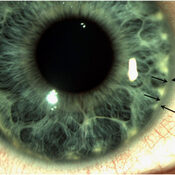
Justification: Neonatal cholestasis is an important cause of chronic liver disease in young children. Late referral and lack of precise etiological diagnosis are reasons for poor outcome in substantial number of cases in India. There is a need to create better awareness among the pediatricians, obstetricians and primary care physicians on early recognition, prompt evaluation and referral to regional center
Process: Eminent national faculty members were invited to participate in the process of forming a consensus statement. Selected members were requested to prepare guidelines on specific issues, which were reviewed by two other members. These guidelines were then incorporated into a draft statement, which was circulated to all members. A round table conference was organized; presentations, ensuing discussions, and opinions expressed by the participants were incorporated into the final draft.
Objectives: To review available published data on the subject from India and the West, to discuss current diagnostic and management practices in major centers in India, and to identify various problems in effective diagnosis and ways to improve the overall outcome. Current problems faced in different areas were discussed and possible remedial measures were identified. The ultimate aim would be to achieve results comparable to the West.
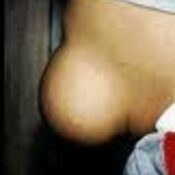
Recommendations: Early recognition, prompt evaluation and algorithm-based management will improve outcome in neonatal cholestasis. Inclusion of stool/urine color charts in well baby cards and sensitizing pediatricians about differentiating conjugated from the more common unconjugated hyperbilirubinemia are possible effective steps. Considering the need for specific expertise and the poor outcome in suboptimally managed cases, referral to regional centers is warranted.
Keywords: Cholestatic jaundice, Neonate, Practice guidelines.
American College of Radiology (ACR), Society for Pediatric Radiology (SPR). ACR-SPR practice guideline for the performance of contrast esophagrams and upper gastrointestinal examinations in infants and children. [online publication]. Reston (VA): American College of Radiology (ACR); 2010. 6 p
1.For a routine esophagram, the patient should not have ingested anything by mouth for a minimum of 2 hours. For upper GI examination, oral feeding should be withheld for a time period appropriate for the patient's age: approximately 2 to 3 hours for neonates and young infants, 4 hours for older infants and children. Adolescents should fast for at least 6 to 8 hours. Emergency examinations may be performed with shorter fasting times.
2.An appropriate medical history should be available, including results of laboratory tests and imaging, endoscopic, and surgical procedures as applicable.Use of a child life specialist and/or parent may be helpful in enabling young patients to cooperate for the examination. Immobilization devices may be helpful in patient positioning. These devices may help limit repeat radiographic exposures and unnecessary radiation dose to patients, parents, technologists, and other personnel.
3.A preliminary "scout" image of the chest and/or abdomen may be useful, depending on the specific clinical concern. Preliminary images should be assessed for calcifications, skeletal abnormalities, anomalies of situs, bowel gas pattern, pneumoperitoneum, evidence of prior surgery, catheters, and monitoring electrodes. A horizontal beam image should be performed if the patient has an underlying condition that might predispose to esophageal, gastric or bowel perforation. Scout images are especially helpful in the workup of neonatal bowel obstruction, since they may influence the choice of initial fluoroscopic study (e.g., upper GI for proximal bowel obstruction and contrast enema for distal small bowel or colonic obstruction).
4. A barium preparation or other contrast medium should be delivered in a manner that is appropriate for the patient's age. Flavoring agents may be added in older patients. Neonates and infants may be fed contrast from a baby bottle with a nipple. Alternatively, a device consisting of a feeding tube or orogastric tube passed through a nipple is sometimes used to deliver the contrast into the mouth; this should be done under careful fluoroscopic control to prevent aspiration. Older infants able to bottle-feed themselves may be allowed to do so. The contrast may be given by straw or taken directly from a cup by an older child. A nasogastric tube, gastrostomy tube, or jejunostomy tube may be used as appropriate. In neonates or young infants with a history of bilious emesis, a nasogastric tube can be placed with tip in the distal stomach to empty the stomach so that a controlled upper GI with a small amount of barium and air can effectively evaluate for malrotation and/or volvulus, using the least amount of barium and time.
5. The amount and type of contrast material given are determined by the child's age and the indications for the study. Barium is the preferred contrast medium for most studies. Nonionic iodinated contrast media may be given to assess the integrity of an esophageal anastomosis, diagnose duodenal obstruction or perforation, or diagnose intestinal malrotation in selected critically ill patients. Iso-osmolar or near iso-osmolar solutions are important in cases in which there is risk of aspiration. Iso-osmolar contrast media are particularly important in critically ill premature neonates and infants to avoid serum electrolyte shifts. Gastrografin, a very highly osmotically active water soluble contrast agent, should not be administered orally in neonates and young infants, because this patient population has a higher risk of gastroesophageal reflux and aspiration, and aspirated hyperosmolar contrast may result in pulmonary edema and a severe chemical pneumonitis.
6.Single-contrast esophagram:The anatomic structure and motility of the entire esophagus should be evaluated fluoroscopically. Appropriate images should be obtained to document normal and abnormal findings. The examination is optimally performed in the lateral and anteroposterior projections, with visualization of the nasopharynx to the gastric fundus.
7. Esophagrams performed in infants with suspected tracheo-esophageal fistula are optimally performed in a controlled manner, with full distension of the esophagus, which can be achieved with normal drinking in patients who drink contrast readily. In patients who do not drink sufficient contrast to distend the esophagus, the contrast can be administered in small amounts initially through a small feeding tube placed in the upper esophagus near the thoracic inlet, with the infant in a right anterior oblique or lateral position. This requires careful fluoroscopic monitoring to prevent aspiration, and monitoring of the contrast as it exits the tubes. If no fistula is identified on the early images, the study may be completed with standard oral administration of contrast. Fluoroscopic observation in the lateral view throughout contrast instillation usually will allow differentiation of contrast in the trachea due to aspiration versus a fistula.
8. Imaging of the esophagus should include an assessment of swallowing in the lateral view, especially if the patient has symptoms suggesting swallowing dysfunction such as coughing and choking and/or gagging during feeding. This should include imaging from the base of the tongue through the upper esophageal sphincter. Modified barium swallow is a more detailed evaluation of the oral, pharyngeal, and upper esophageal phases of swallowing, usually performed in conjunction with a speech pathologist or occupational therapist. Variable thickness barium contrast may be used to assess the effect of viscosity of the fluid on swallowing function.
9. Double-contrast esophagrams are seldom performed in pediatric patients, but they may help to evaluate mucosal integrity in adolescents.
10. Fluoroscopic assessment of swallowing and the anatomic structure and motility of the entire esophagus, stomach, and duodenum should be performed, and appropriate images should be obtained to document normal and abnormal findings. Suggested images include frontal and lateral views of the barium-distended esophagus, stomach, and duodenum and images of the partially filled esophagus. Initial passage of contrast through the duodenum should be observed directly with fluoroscopy to confirm the position of the duodenojejunal junction (DJJ). This can be documented with serial multiple fluoro-capture images or fluoroscopy video capture, where available. On the first upper GI examination in an infant or child, the position of the DJJ should be documented on both frontal and lateral positions to diagnose or exclude malrotation. The lateral view is important to assure the retroperitoneal position of the normally rotated duodenum and the normal height of the duodenal-jejunal junction at the level of the duodenal bulb; additionally, the frontal view assures the normal position of the DJJ, at or to the left of the left pedicle of the vertebral bodies. A sufficient number of digital spot or last image hold images or fluoroscopy video captures to adequately demonstrate or exclude pathology should be obtained.
ESPGHAN GUIDELINES ON CELIAC DISEASE, SHORT SUMMARY
There is a strong genetic predisposition to CD with the major risk attributed to the specific genetic markers known as HLA-DQ2 and HLA-DQ8. LOE: 1.
The main role of HLA-DQ typing in the diagnosis of CD is to exclude the disease. LOE: 2.
HLA-DQ2 and/or HLA-DQ8 have poor specificity for CD (median 54%), indicating a low positive predictive value for CD.LOE: 2.
groups at risk for CD. A negative result for HLA-DQ2/HLA-DQ8 renders CD highly unlikely in these children, and hence there is no need for subsequent CD antibodies testing in such individuals. LOE: 2.
Offer HLA-DQ2 and HLA-DQ8 typing in patients with uncertain diagnosis ofCD, for example, in patients with negativeCDspecific antibodies andmild infiltrative changes insmall-bowel specimens. Negative results render CD highly unlikely in these children.
HLA-DQ2/HLADQ8 typing in children WITHOUT intestinal biopsies to add strength to the diagnosis.
In subjects with normal serum IgA values for age, a positive IgA class EMA result or a positive IgA class anti-TG2 antibody result is considered to be a CD-relevant antibody positivity. In the case of IgA deficiency, a positive IgG class EMA result, a positive IgG class anti-TG2 antibody, or a positive IgG class anti-DGP antibody is diagnostically relevant. LOE: 1.
For blood anti-TG2 antibody tests that use calibration curves to express antibody concentration, values exceeding 10 times ULN may be denoted as high antibody positivity. LOE: 3.
It is more likely that CD is present if the EMA result is positive than if another CD antibody result is positive. LOE: 1.
Isolated positivity for anti-TG2, especially in the low positivity range, can occur in conditions that are unrelated to CD, such as other autoimmune conditions, infections, tumours, or tissue damage. LOE: 1.
High concentrations of anti-TG2 antibodies in blood (as defined in statement 2.3.5) predict villous atrophy better than low positive or borderline values. LOE: 2
The evaluation of rapid tests is less reliable if done by untrained or laypeople.LOE 1
Anti-TG2 antibody or EMA testing from a blood sample has a higher accuracy than antibody testing against DGP, unless special patient characteristics are present (IgA deficiency, age younger than 2 years).LOE: 1.
Tests for the detection of IgG or IgA antibodies against native gliadin (conventional gliadin antibody test) are neither sufficiently sensitive nor sufficiently specific for the detection of CD. LOE: 1.
Tests for the detection of CD antibodies of any isotype (IgG, IgA, secretory IgA) in fecal samples are unreliable. LOE: 3.
For initial testing in symptomatic patients, a quantitative test detecting IgA class anti-TG2 or EMA from a blood sample is recommended. If total serum IgA is not known, measurement is recommended. In subjects with either primary or secondary humoral IgA deficiency, at least 1 additional test measuring IgG class CD antibodies (IgG anti-TG2, IgG anti-DGP, or IgG EMA, or blended kits for both IgA and IgG antibodies) is recommended.
Children found to test positive for CD-specific antibodies should be evaluated by a paediatric gastroenterologist to prove or to exclude the presence of CD.
Histological assessment may be omitted in symptomatic patients (see list in Who to Test) who have high IgA anti-TG2 levels (10 times above ULN), verified by EMA positivity, and are HLADQ2 and/or HLA-DQ8 heterodimer positive.
If anti-TG2 antibodies are positive only in low concentrations and EMA testing is negative, then the diagnosis of CD is less likely. A small intestinal biopsy should be performed to clarify whether CD is present.
In seronegative patients with strong clinical suspicion of CD, small-intestine biopsies are recommended.
Biopsies should be taken from the bulb (at least 1) and from the second or third portion of the duodenum (at least 4).
Gluten challenge is not considered mandatory, except under unusual circumstances. These circumstances include situations in which there is doubt about the initial diagnosis, including patients with no CD-specific antibodies before starting a GFD.
Gluten challenge should be performed under medical supervision, preferably by a paediatric gastroenterologist.
HLA typing and assessment of duodenal histology should be considered before gluten challenge is instituted
----------------------------------------------------------------------------------------------------------------------------------
Probiotics As Pharmacobiotics:re –naming ceremony.
WHY WE SHOULD CONSIDER AS DRUG?
- virulence factors from pathogens may be acquired by probiotic strains.
- Arch Microbiol. 2011 Mar ; 193(3): 157-68
WHAT'S OUR WORRY ?
no clear international or local legislation .most of the tested products are not in conformity with international guidelines.
WHAT ARE PROBIOTICS ?
- produce lactic acid.
- Lactobacillus rhamnosus GG (LGG),
- Bifidobacterium lactis,
- the yeast Saccharomyces boulardii.
- Streptococcus thermophilus.
Pediatrics 2010;126:1217–1231 Clinical ReportProbiotics and Prebiotics in Pediatrics
WHAT PREBIOTICS ARE STUDIED?
- fructooligosaccharides
- inulin,galacto-oligosaccharides (GOSs),
- soybean oligosaccharides
- Prebiotics :STUDIES?
- Pediatrics 2010;126:1217–1231 Clinical ReportProbiotics and Prebiotics in Pediatrics
Any INDIAN STUDY?
Safety concerns with the use of probiotics in infants and children who are
- immunocompromised,
- chronically debilitated,
- or seriously ill with indwelling medical devices.
- Pediatrics 2010;126:1217–1231 Clinical ReportProbiotics and Prebiotics in Pediatrics
ACUTE GASTROENTERITIS AND PHARMACOBIOTICS
Pxdoes not support the routine use of probiotics to prevent infectious diarrhea.
Rx use of probiotics reduces diarrhea duration by 1 day. benefit is strain dependent. LGG is the most effective. dose >10 billion.More Useful more for rotavirus diarrhea
ANTIBIOTIC ASSOCIATED DIARRHOEA AND PHARMACOBIOTICS
PX:Some evidence to support the use of probiotics in AAD RX:NO EVIDENCE IN AAD
- STRAIN IN AAD :LGG, B lactis, S thermophilus, and S boulardii
- Pediatrics 2010;126:1217–1231 Clinical ReportProbiotics and Prebiotics in Pediatrics
PHARMACOBIOTICS ; WHAT WE SHOULD KNOW ?
1.it should not be assumed that efficacy with one strain applies to another.
2.Not uniform.
3.same strain will not be equally effective in a different indication.
4.efficacy with probiotics in any indication is likely to be modest.
5.supplements not substitutes
6.more is not always better.
7.combining probiotics = foolish.
8.dietary–probiotic interactions are there respect them.
Gastroenterol Clin N Am 39 (2010) 721–726
What are TURBO PHARMABIOTICS?
- mucosal delivery of anti-inflammatory cytokines.
- Crohn disease.
- Braat H, Rottiers P, Hommes DW, et al. A phase I trial with transgenic bacteria expressing interleukin-10 in Crohn’s disease. Clin Gastroenterol Hepatol 2006; 4:754–9.
---------------------------------------------------------------------------------------------------------------------------------------------
EB-PGHN AND IAP-PICU: GUIDELINES & PROTOCOL :
APPROACH TO GI BLEED IN CHILDREN :POSITION PAPER 2010.FOR FREE PAPER E-MAIL : pedgihep@yahoo.com
--------------------------------------------------------------------------------------------------------------------------------------------
EB-PGHN CLINICAL GUIDELINES Current RE-view on intestinal microbiota in neonates:
The preterm gut is essentially a fetal gut, expecting conditions of the intrauterine environment. This includes absence of bacteria, absence of food substrate, low oxygen consumption due to the role of the placenta as the primary organ of nutrition, and presence of amniotic fluid with proteins and hormones which may have a role in maturation. Rapid colonization of the intestine after birth with a variety of microbial species is a normal part of development. So begins a complex cross-talk between the gut and its microflora with implications for immune, inflammatory, and allergic responses. It is not merely the existence of bacteria but the balance of bacteria that affects a variety of signaling pathways and immune responses.
The interaction between the intestine and bacterial microflora is a complex relationship with risk and benefit for the host. It is sobering to realize that of the estimated 1014 cells in the human body, only about 10% belong to the host and the remaining 90% represent the microbiota living in or on the host, containing a genome or “microbiome” collectively more extensive than our own . The human intestine is populated with more microorganisms than any other organ, and in fact some have considered the microflora itself equivalent to an ancillary body organ based on weight, genetic content, cellular content, and metabolic activity of these organisms. Many are difficult to grow in culture and are only now being identified via 16S ribosomal DNA polymerase chain reaction fingerprinting. In a healthy host, beyond merely colonizing the gut awaiting entry into the bloodstream, these microorganisms have a significant impact on intestinal health and gut
function.
Colonization begins at birth with bacteria that represent maternal vaginal and colonic flora in vaginally delivered infants. This flora contains many facultative anaerobic bacteria such as Enterobacteriae, Enterococci, and Staphylococci. As the number of aerobic and facultative anaerobic organisms increase, oxygen is consumed, allowing for colonization by anaerobic organisms . Thereafter colonization is affected by feeding, with bacteria introduced via either breast milk or formula feeds along with factors, particularly in breast milk, which influence colonization .Weaning from breast milk, the introduction of solid foods, and the environment further affect colonization. This ultimately leads to complete adult colonization by 2 years of age with 400–1000 different species and a stable pattern that is unique for each individual. There is evidence that different organisms can affect different intestinal functions and it is likely that the
synergy of the diversity of intestinal microbiota is important for the balance of intestinal health and appropriate control of pathogenic organisms.
Microbial flora is determined not only by the organisms to which the intestine is exposed, but also by intrinsic intestinal host defense mechanisms. The intestine has many physical barriers to bacteria including peristalsis, gastric acid, proteolytic enzymes, intestinal mucus, cell surface glycoconjugates, and tight junctions between intestinal epithelial cells. These are designed to limit bacteria to the gut lumen and prevent attachment and translocation across the intestinal epithelium. The intestine also has a complex system of immunologic host defense and biochemical factors designed to limit the growth of organisms that breach the physical barrier. This includes intestinal T and B lymphocytes, sIgA, human defensins secreted by Paneth cells, and intestinal trefoil factor secreted by the intestinal epithelium itself.
The intestine has developed multiple means of sampling and interacting with its microflora so that commensal bacteria can be recognized and tolerated while pathogenic organisms trigger inflammatory and immune responses. Three cell types can sample the microbial milieu: M cells, surface enterocytes, and dendritic cells.
In the nondiseased mature state there is tight regulation of protective inflammatory and allergic host responses. Under baseline conditions the intestinal mucosa is in a constant “suppressed” state due to increased transforming growth factor beta (TGFβ) and IL-10 produced by regulatory T cells and Th3 cells If bacteria breech the mucosal barrier, allowing presentation of bacterial products in the context of TLR, Th1 responses are triggered, TH2 responses are suppressed, and appropriate inflammatory responses are induced to contain the bacterial insult. When no response is required, Th2 cells produce IL-4 and IL-10, which appropriately suppress Th1 responses, while Th1 cells produce IFNγ and IL-12, which appropriately suppress Th2 responses, returning the balance to its baseline state. Dysregulation can lead to conditions of inappropriate responses to bacteria such as inflammatory bowel disease, or to food antigens such as food allergies
and celiac disease.
Necrotizing enterocolitis is an inflammatory bowel necrosis that primarily affects preterm infants after the initiation of enteral feeds. While the exact pathophysiology is poorly understood, the primary risk factors are prematurity, bacterial colonization, enteral feeds and altered intestinal blood flow. It is known that the fetal intestine is exposed to amniotic fluid containing hormones and peptides that may have a role in intestinal maturation. At this stage, the fetal intestine is normally protected in its sterile environment and may not be prepared to respond to bacterial interaction. The preterm gut may not have completed maturation when colonized by bacteria and initially fed, potentially placing it at higher risk for NEC. One possible hypothesis is that intestinal injury in NEC may be the result of synergy in which enteral feeding results in colonization of the uniquely susceptible premature intestine with pathogenic bacteria, resulting in
mucosal damage leading to an exaggerated inflammatory response.
Certainly there are concerns and issues of safety in using probiotics. First, do we really want to give live organisms to our relatively “immunocompromised” preterm patients? Cases of Lactobacillus sepsis have been documented and prompt caution.Interestingly some protective probiotic effects such as proteasome inhibition and hsp production can be obtained using bacteria-free probiotic-conditioned media.
Another option is the administration of prebiotics. Prebiotics are non-digestible food ingredients that benefit the host by selectively stimulating the growth or activity of a limited number of bacterial species already resident in the intestine. For example, lactulose is neither metabolized nor absorbed in the intestine and has been shown to decrease colonic pH by increasing production of organic acids, thus improving the environment for Lactobacillus and Bifidobacteria growth. It has been shown in IL-10 knockout animals at risk for colitis that increasing Lactobacillus species in the colon by prebiotics results in a concomitant decrease in levels of adherent and translocated bacteria, resulting in protection against colitis similar to results obtained by administering Lactobacillus directly.
While the concept of prebiotics is intriguing as a means to support the growth of beneficial organisms in a probably cost-effective manner, without actually administering live bacteria, and thus with presumed minimal side-effects, clinical studies have not fully
evaluated the efficacy of this intervention.
Another concern is efficacy and means of administration. In the clinical studies of the effect of probiotics on preterm infants numbers were small and populations used may or may not be generalizable. Further, no mechanism of action, dosage, or timing specifics are known. One problem is that different studies of probiotics have used different organisms. Multiple studies have shown that different organisms have different effects, and even different strains of the same organism may behave differently.
Microarray studies of intestinal epithelial cells from germ-free mice colonized with organisms including Bacteroides thetaiotaomicron, Bifidobacterium infantis, E. coli, or complete microflora, demonstrated bacteria-induced effects on gene expression affecting nutrient absorption, barrier function, intestinal mucus, gut motility, and toxin metabolism. However, not all bacteria affected all, or had the same effect, on all genes.
Molecular techniques to evaluate mechanisms of action of specific probiotics and document survival and colonization of organisms after their administration will allow more specific application of probiotic therapy. Perhaps a combination of probiotics to produce a synergy of effects is best.
refrences:
1.The Intestinal Microbiota and the Microbiome Erika C Claud, MD, W. Allan Walker, MD.
2.Hooper LV, Bry L, Falk PG, et al: Host-microbial symbiosis in the mammalian intestine: exploring an internal ecosystem. Bioessays 1998; 20(4):336-343.
3.Bourlioux P, Koletzko B, Guarner F, et al: The intestine and its microflora are partners for the protection of the host: report on the Danone Symposium “The Intelligent Intestine”, held in Paris, June 14, 2002. Am J Clin Nutr 2003; 78(4):675-683
4.Karlsson H, Larsson P, Wold AE, et al: Pattern of cytokine responses to gram-positive and gram-negative commensal bacteria is profoundly changed when monocytes differentiate into dendritic cells. Infect Immun 2004; 72(5):2671-2678.
5.Orrhage K, Nord CE: Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr Suppl 1999; 88(430):47-57
6.Fehervari Z, Sakaguchi S: CD4+ Tregs and immune control. J Clin Invest 2004; 114(9):1209-1217
7.Prioult G, Nagler-Anderson C: Mucosal immunity and allergic responses: lack of regulation and/or lack of microbial stimulation?. Immunol Rev 2005; 206:204-218.
8.Rinne MM, Gueimonde M, Kalliomaki M, et al: Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol Med Microbiol 2005; 43(1):59-65.
9.Land MH, Rouster-Stevens K, Woods CR, et al: Lactobacillus sepsis associated with probiotic therapy. Pediatrics 2005; 115(1):178-181
10.Cukrowska B, LodInova-ZadnIkova R, Enders C, et al: Specific proliferative and antibody responses of premature infants to intestinal colonization with nonpathogenic probiotic E. coli strain Nissle 1917. Scand J Immunol 2002; 55(2):204-209.